Heat development in concrete is a very complex and one of the most extensively researched topics (both at the academic level and the industrial level). To simplify this process, heat development over time can be separated into five distinct phases. The heat profile can change depending on the type of cement. The article only touches on the typical hydration for Type I cement. The basic of the heat development is graphically presented in the figure below.
Hydration
When portland cement is mixed with water its chemical compound constituents undergo a series of chemical reactions that cause it to harden (or set). These chemical reactions all involve the addition of water to the basic chemical compounds listed in Table 1. This chemical reaction with water is called “hydration”. Each one of these reactions occurs at a different time and rate. Together, the results of these reactions determine how portland cement hardens and gains strength.
Table 1: Portland cement composition
Portland Cement Phases | Chemical Formula | Shorthand Notation (Abbreviation) |
Dicalcium silicate | 2CaO×SiO2 | C2S |
Tricalcium silicate | 3CaO×SiO2 | C3S |
Tricalcium aluminate | 3CaO×Al2O3 | C3A |
Tetracalcium aluminoferrite | 4CaO×Al2O3×Fe2O3 | C4AF |
- Tricalcium silicate (C3S). Hydrates and hardens rapidly and is largely responsible for initial set and early strength. Portland cements with higher percentages of C3S will exhibit higher early strength.
- Dicalcium silicate (C2S). Hydrates and hardens slowly and is largely responsible for strength increases beyond one week.
- Tricalcium aluminate (C3A). Hydrates and hardens the quickest. Liberates a large amount of heat almost immediately and contributes somewhat to early strength. Gypsum is added to portland cement to retard C3A hydration. Without gypsum, C3A hydration would cause portland cement to set almost immediately after adding water.
- Tetracalcium aluminoferrite (C4AF). Hydrates rapidly but contributes very little to strength. Its use allows lower kiln temperatures in portland cement manufacturing. Most Portland cement color effects are due to C4AF.
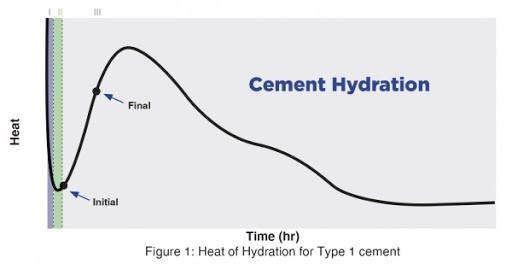
Phase I: Pre-Induction
Shortly after water comes into contact with cement, there is a sharp increase in temperature, which happens very quickly (within a couple of minutes). During this period, the primary reactive phases of the concrete are the aluminate phases (C3A and C4AF). The aluminate and ferrite phases react with the calcium and sulfate ions to produce ettringite, which precipitates on the surface of the cement particles. During this phase, to a lesser extent, the silicate phases (mainly C3S) will also react in very small fractions compared to their total volume and form a very thin layer of calcium-silicate-hydrate (C-S-H).
Phase II: Dormant Period
This phase is also known as the induction phase. During this period, the rate of hydration significantly slows down. This is believed to be due to the precipitation of the aforementioned compounds on the surface of the cement particles, which leads to a diffusion barrier between these particles and water. Nevertheless, there is significant debate regarding the physical and chemical properties behind the occurrence of this stage and the methods to predict it.
During this period fresh concrete is transported and placed. At this time, the concrete has not yet hardened and is still workable (plastic and fluid). The length of the dormant period has been shown to vary depending on multiple factors (cement type, admixtures, w/cm, etc.). The end of the dormant period is typically characterized by the initial set.
Phase III and IV: Strength Gain
In this phase, the concrete starts to harden and gain strength. The heat generated during this phase can last for multiple hours and is caused mostly by the reaction of the calcium silicates (mainly C3S and to a lesser extent C2S). The reaction of the calcium silicate creates a “second-stage” calcium silicate hydrate (C-S-H), which is the main reaction product that provides strength to the cement paste. Depending on the type of cement, it is also possible to observe a third, lower heat peak from the renewed activity of C3A.
Phase V: Steady State
At this point, the temperature of the concrete stabilizes with the ambient temperature. The hydration process will significantly slow down but will not completely stop. Hydration can continue for months, years, or even decades provided there is sufficient water and free silicates to hydrate. However, strength gain will be minimal during this period.
Reference & Further Readings
- Mindess, S. and Young, J.F. (1981). Concrete. Prentice-Hall, Inc. Englewood Cliffs, NJ.
- A.M. Neville (2003). Properties of Concrete. Pearson Education Limited